Abstract
Background While recently published analyses have characterized findings from comprehensive genomic analyses (PMID: 29713087, 29641966) or targeted sequencing (PMID: 32187361) performed on tumor biopsies (bx) from patients (pts) with newly-diagnosed diffuse large B cell/high grade B cell lymphoma (DLBCL/HGBL), analyses of mutations (mut) found in tumor bx from pts with relapsed/refractory (R/R) DLBCL/HGBL have been limited and largely descriptive in nature (PMID: 26647218, ICML 2017 #294). We aim to describe the clinicopathologic characteristics including tumor mutation profile, as well as therapy (tx) received and survival outcomes for pts with R/R DLBCL/HGBL who were treated with palliative-intent at our institution.
Methods Pts diagnosed with DLBCL/HGBL who had at least one prior tx and experienced R/R disease between 2015-2021, who had tumor bx performed immediately prior to the receipt of palliative-intent tx which underwent clinical laboratory mutation analysis (CLMA) performed at the University of Pennsylvania with one of three lymphoma sequencing panels, were retrospectively analyzed. Tx was given at the discretion of treating physician. Result of fluorescence in situ hybridization for MYC, BCL2 and BCL6 as well as immunohistochemical staining for MYC and BCL2 were from current bx or prior bx if not performed on current bx. Overall survival (OS) was defined from time of bx of R/R disease and date of death or last follow-up while alive, with cut-off at 7/1/22. Univariate Cox regression analysis for OS from time of bx was performed.
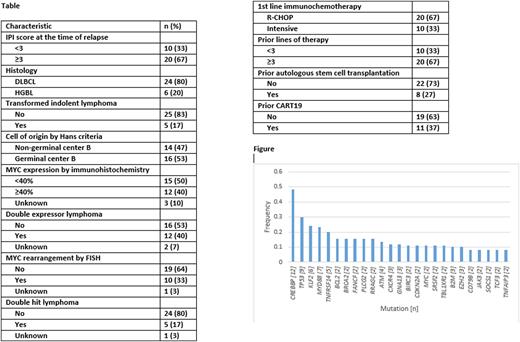
Results CLMA was performed on 35 pt tumor bx with 39 successful assays (86% success rate) with median turnaround time for successful result of 19.5 days. Baseline clinicopathologic and treatment characteristics can be seen in the Table. Sequencing panels identified CREBBP as the most frequent mutation present in the tissue samples, occurring in 12 (48%) of samples tested for CREBBP, followed by TP53 occurring in 9 (30%) and MYD88 occurring in 7 (23%). A histogram of all detected mutations with n≥1 is depicted in the Figure. Next-line of tx was classified as polatuzumab-containing (n=8), small molecule inhibitor-containing (7), tafasitamab-containing (4), bispecific antibody (n=3) and other (n=8). With a median length of follow-up of 19.1 months, the median OS for the entire population was 18.1 months. Characteristics listed in the Table which were predictive of OS with P<0.10 on univariate Cox regression analysis were International Prognostic Index Score at the time of relapse ≥3 vs <3 (hazard ratio [HR] 3.0, 95% confidence interval [CI] 0.85-10.3, P=0.09), HGBL vs DLBCL histology (HR 6.8, 95% CI 2.3-20.2, P=0.001), MYC rearrangement vs not (HR 2.4, 95% CI 0.9-6.6, P=0.08) and double hit lymphoma vs not (HR 4.8, 95% CI 1.6-14.5, P=0.005). None of the above-mentioned mut predicted for OS.
Conclusions In this pt population with R/R DLBCL/HGBL receiving palliative-intent tx, mut identified by CLMA revealed CREBBP, TP53 and MYD88 occurred frequently these nor other mut predicted for OS. Although a relatively small sample population without uniform tx, this study demonstrates the presence of recurring mut which could be used to guide palliative tx decisions. A study with a larger cohort of pts whose tumor bx undergo CLMA with a single comprehensive mut panel could be valuable in further understanding the predictive value of tumor biopsy mut this pt population based on tx received.
Disclosures
Schuster:Novartis: Consultancy, Research Funding; Regeneron: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Research Funding; Merck: Research Funding; DTRM: Research Funding; Juno Therapeutics: Research Funding; Abbvie: Research Funding; Adaptive Biotechnologies: Research Funding; Nordic: Consultancy; Morphosys: Consultancy; MustangBio: Consultancy; Incyte: Consultancy, Research Funding; Genetech/Roche: Consultancy, Research Funding; Janssen: Consultancy; Legend Biotech: Consultancy; Loxo: Consultancy; Acerta: Consultancy; BiGene: Consultancy; Celgene: Consultancy, Research Funding; Nanovector: Consultancy; TG Therapeutics: Research Funding. Dwivedy Nasta:Pharmacyclics: Research Funding; Roche: Research Funding; Rafael: Research Funding; FortySeven/Gilead: Research Funding. Gerson:Genentech: Consultancy; Abbvie: Consultancy; Loxo Oncology: Research Funding. Barta:Acrotech: Honoraria; Daiichi Sankyo: Consultancy; Seagen: Honoraria; Kyowa Kirin: Consultancy, Honoraria; Janssen: Other: Independent Data Monitoring Committee member; Affimed: Consultancy. Svoboda:TG: Research Funding; SEAGEN: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Merck: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Consultancy; BMS: Consultancy, Research Funding; Atara: Consultancy; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; ADCT: Consultancy. Chong:Juno/BMS: Consultancy; Novartis: Consultancy; Beigene: Consultancy; KITE: Consultancy; Tessa: Consultancy. Lim:EUSA Pharma: Honoraria. Landsburg:Morphosys: Membership on an entity's Board of Directors or advisory committees; Epizyme: Membership on an entity's Board of Directors or advisory committees; Curis, Inc: Research Funding; Calithera: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Triphase: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal